
Engineering Document Management for Life Sciences
Streamline compliance and system validation.
Adept gives Life Sciences companies centralized access to your most important documents in a secure, collaborative environment that simplifies compliance and system validation for FDA 21-CFR Part 11 and EU GMP Annex 11. Connect your teams with Adept and you will reduce risk, improve efficiency, and lower your costs.
"What do we get from Adept? The big thing is my risk profile is vastly improved, and now I have a validated system. We now have version control. We have a centralized source of truth. The capability to set up workflows for different scenarios, like GmP, Safety, or Engineering."
Join other leaders in Life Sciences.












































System validation services.
- System validation for FDA 21-CFR Part 11 and/or EU GMP Annex 11
- Leverage proven system validation experience with Adept
- Adept environment pre-validated and pre-configured
- Customer-specific validation documentation
- Save time and minimize impact to your resources
- Optional quarterly risk assessment and validation testing

One source of truth.
Centralized document control.
Centrally manage plant, facility, and equipment documentation to align your engineering, maintenance, operations and construction teams. Make your documents more accessible, secure, and traceable. Improve data quality and avoid unplanned downtime and safety issues. Provide enterprise visibility to plant and project information.
Drive standards and best practices.
Adept automates tasks like version control, file naming, workflow processes, approval validation, print stamps, watermarks, and an audit trail. With Adept you can drive standards, automate processes, and deploy best practices across your plants, facilities and the enterprise. You’ll simplify system validation and compliance for FDA 21-CFR Part 11 and EU GMP Annex 11.
"We had tried for several years to get funding for this, but were not successful until we had a near miss issue where there was some excavation to take place. And fortunately, the people that were out there questioned the drawing and they discovered it was an older version and there had been other work in the area in the past. If they had dug where they were planning to, it would've resulted in digging up power lines, which we consider a bad thing."
Security, control, and traceability.
- Ensure the availability, integrity and confidentiality of your data
- Vault your designs and documents in a safe and secure environment
- Control user and group access to documents with the granularity you need
- Maintain an extensive, searchable document audit trail
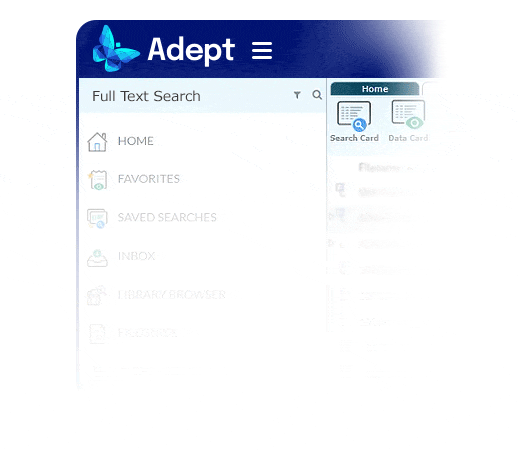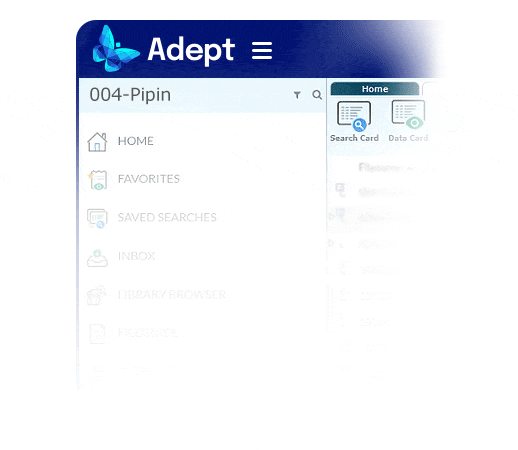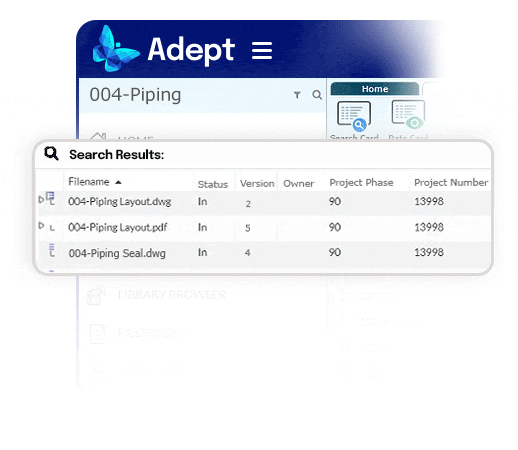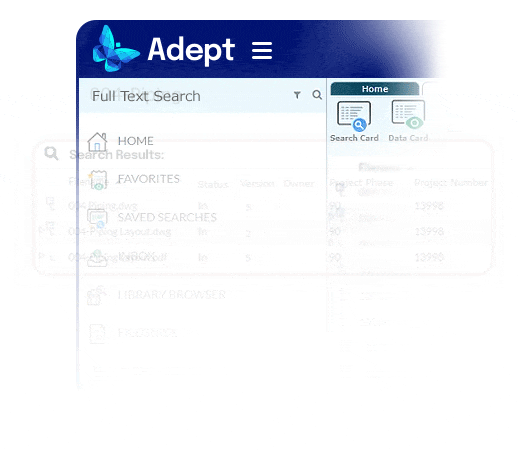
The right document to the right person at the right time.
- Recover a day per week per person by eliminating time wasted searching
- End the reliance on complex folder systems across enterprise servers
- Empower fast access to documents, no matter where they're stored
- Use full text and metadata search, or browse a flexible tree structure
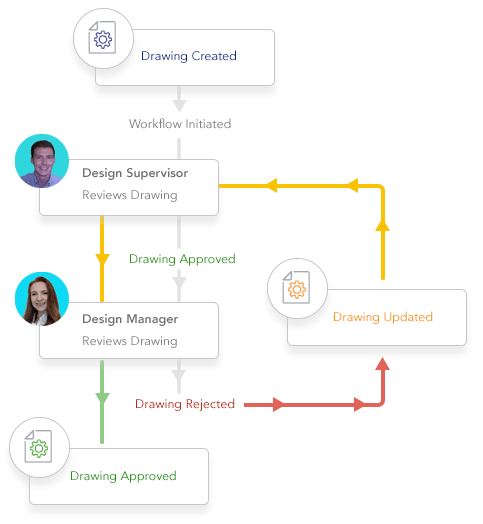
Automate workflow and engineering change.
- Crush bottlenecks and delays from manual workflow methods
- Streamline engineering change and other approval processes
- Keep everything on track with automated notifications and alerts
- Avoid duplication of documents that cause mistakes
- Harmonize as-built documentation with capital projects to avoid mistakes
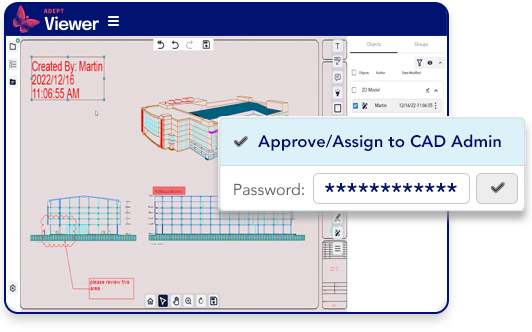
Validate approvals and ensure digital signatures.
- Require password authentication for document approvals
- Ensure digital approval signatures
- Maintain an audit trail of all approval processes
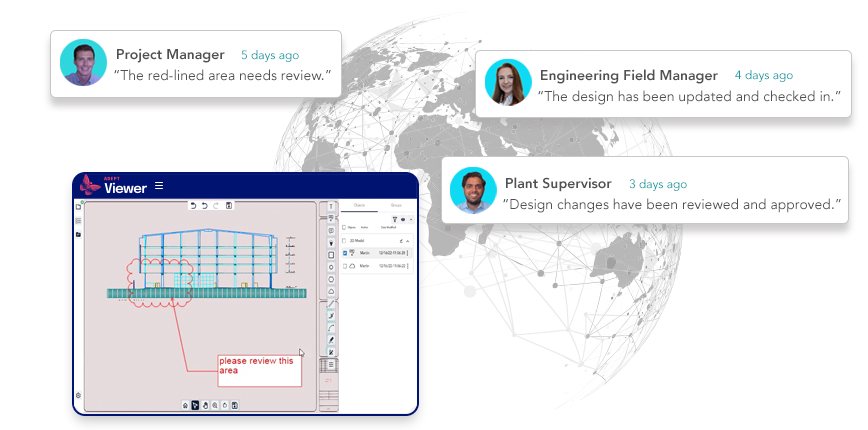
Simplify design visualization and markup for CAD and office files.
- Centralized visualization for hundreds of formats
- Easy digital markup simplifies design reviews and feedback
- Supports 2D and 3D CAD, Word, Excel, PDF, and more
- No CAD license required
Automate digital print stamps and watermarks.
- Ensure controlled documents have a watermark or print stamp whenever viewed, printed or published to PDF
- Indicate relevant status and date information to keep everyone aligned, even across time zones


Streamline document transmittals.
- Automate transmittal creation and management
- Transmittal forms and cover sheets are customizable
- Find version-correct documents with ease
- Automatically include reference files
- Maintain an auditable record of what was sent and when
Improve engineering project handover quality with Adept.
- Receive higher quality documentation faster
- Receive construction as-built markups integrated with your engineering handover package
- Startup sooner and have what you need to maintain the plant or facility
- Review handover metadata quality compliance throughout the project

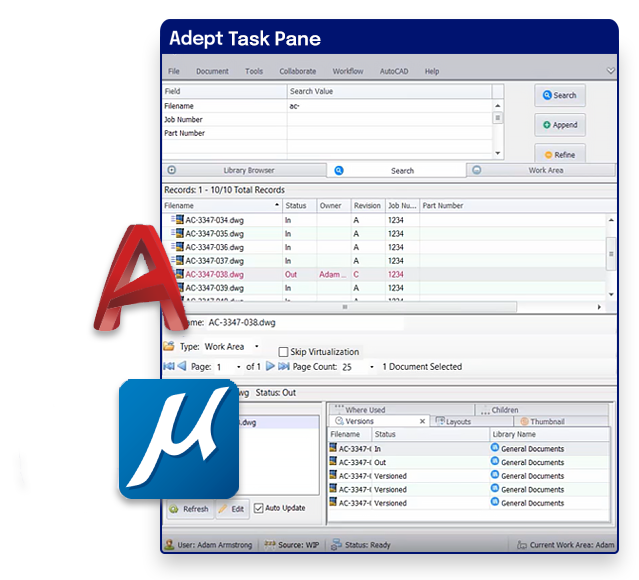
CAD integrations to streamline design.
- Leverage deep integrations with AutoCAD, MicroStation, and Inventor
- Use Adept inside your CAD tool
- Extract all available text for full text search
- Check in/out for version control
- Extract CAD properties into Adept for centralized access and reporting
- Keep 2D and 3D references intact and see where-used and composed-of details
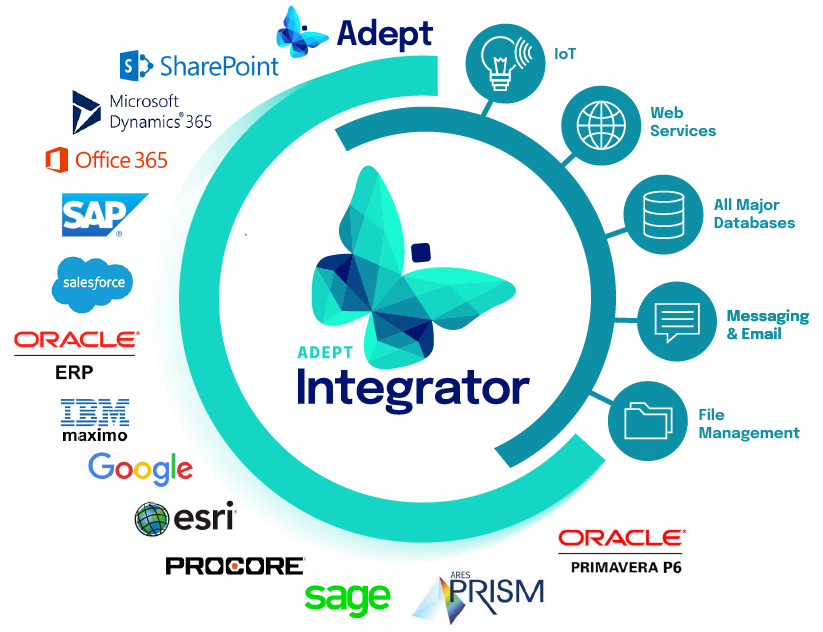
Integrate with CMMS, AIM, ERP, SharePoint and more.
- Give easy access to as-built technical documentation from other systems
- Automate data flows and work processes across business applications
- Eliminate redundant data entry

Go paperless.
- Give your workforce immediate access to the right information
- Improve collaboration and innovation
- Gain traceability with version control and an audit trail
- Enable centralized visibility to information
- Make security and compliance better and easier
- Protect your historic documents from water events, insects and other destructive elements by managing a digital original
- Help the environment



